Summary
Industry: Biotech
Headquarters: Basel, Switzerland
Partnership period: May 2019 – Ongoing
Team size: 15 experts
Software product: Medical device simulators
Expertise delivered: Dedicated Development Team, Java Development Services, Quality Assurance.
Challenge
Our client Roche is a Basel-, Switzerland-headquartered pharmaceutical and BioTech multinational, whose history spans more than 126 years. They are the world’s #1 pharmaceutical company and a leading provider of cancer treatments globally. Roche employs more than 100,000 people in 150 countries.
The client required technical assistance in several important areas. We became the provider of choice for the project through a referral by one of Roche’s technical officers, who had previously worked for a major eCommerce client of ours.
The primary area in which our client needed assistance was developing software for Roche’s DNA-sequencing machine. This machine analyzes multiple DNA samples concurrently. As a person takes a blood test in a lab, the related data is submitted to a system called Connecting Software. The obtained DNA sample is then submitted to the DNA-sequencing machine that performs the DNA-sequencing process. Next, the sequenced DNA samples are transferred to other machines to be further analyzed automatically, or to doctors and scientists, who analyze them manually.
Any new APIs that are added to the system’s embedded software to enable its interactions with the controlling software, need to be tested to make sure that they work properly. However, there is a risk of getting the client’s hardware damaged if there happen to be any incompatibilities between this hardware and any of the newly added features. Because of this, the client engaged us to develop simulator software that would allow virtually testing the features that are added to their DNA-sequencing machine.
The second service Roche engaged us to provide was the remote testing of the DNA-sequencing machine per se.
In addition to the testing of Roche’s DNA-sequencing machine and development of the related simulator software, our experts are also engaged in several more activities as part of the сlient’s in-house team. In particular, such activities include migrating tests from the legacy testing framework to a new one and helping further enhance the product.
Solution
At first, the project team we provided was composed of only 2 experts. In the spring of 2020, this team was significantly expanded and kept on gradually growing afterwards. By April 2022, SPD Technology’s project team had grown to include 15 experts.
The project is currently ongoing. As of February of 2023, 10 members of our project team are involved in testing the client’s software and developing medical device simulators for their DNA-sequencing machine that performs Nanopore DNA sequencing.
Two of our experts are working on the Connecting Software system, and another two – a Java developer and a frontend developer – are engaged in developing other software. Besides, two members of SPD Technology’s project team are converting tests from the client’s legacy framework to the one that has been chosen as its replacement. We also have a Product Owner, who is adding new features to the product.
While testing the client’s DNA-sequencing machine, our experts determine the adjustments that need to be made to the system’s software or hardware to achieve the maximum efficiency of the interactions between them.
Initially, the remote testing of the client’s embedded software posed a significant challenge. We had to work with a liaison in the client’s U.S. laboratory, who would monitor the machine’s response to certain actions and send reports to our testing team in Ukraine. This approach had remained productive for a while, but shortly became inconvenient as the team grew.
In order to optimize the process, the client’s Senior Software Engineering Manager installed a number of webcams on the DNA-sequencing machine and launched online streaming. This allowed our team to see how the machine was responding to changes in the software. It also became possible for them to run tests at any time of the day.
In addition, this solution has helped us turn the problem of a 10-hour time difference into an advantage. It has enabled our team to conduct testing even when the on-site team is not working.
Also, we’ve adjusted our Agile processes, and made our regular meetings maximally productive by excluding those people, who are not needed on the calls.
Don't have time to read?
Book a free meeting with our experts to discover how we can help you.
Book a MeetingTo eliminate the testing-related risks, we have developed medical device stimulators (also referred to as “stubs”). This software emulates the interactions between any newly developed functionality and the hardware of the DNA-sequencing machine. Thus, it allows testing the features being added safely. Besides, our stubs help achieve time savings for the client: 6 or 7 teams at Roche can reuse them to test some other software on other, similar machines.
To improve the testing process, our team has also developed a Software Quality Assurance (SQA) Dashboard.
This application allows monitoring the testing progress by collecting and storing testing reports for multiple projects. It includes such parameters as the test execution time, reasons for test failures, number of failed tests, and so on. It is possible to use the dashboard for any project at Roche, and 7 of their development teams have been using the software for a while now.
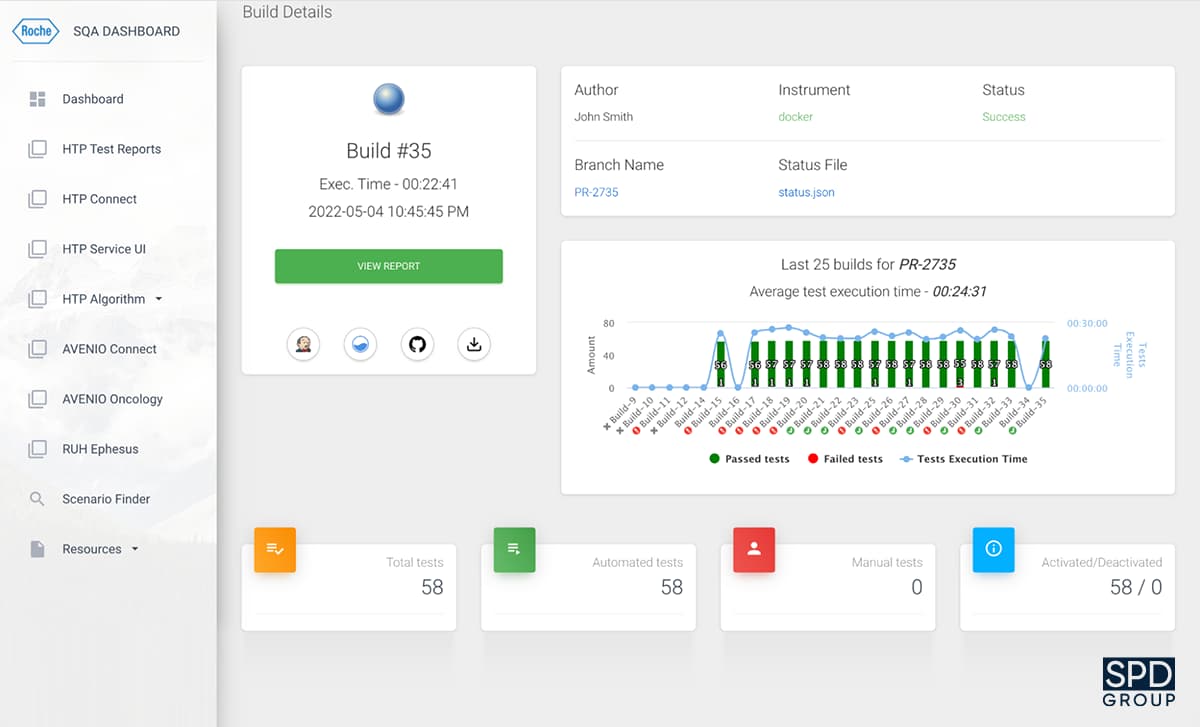
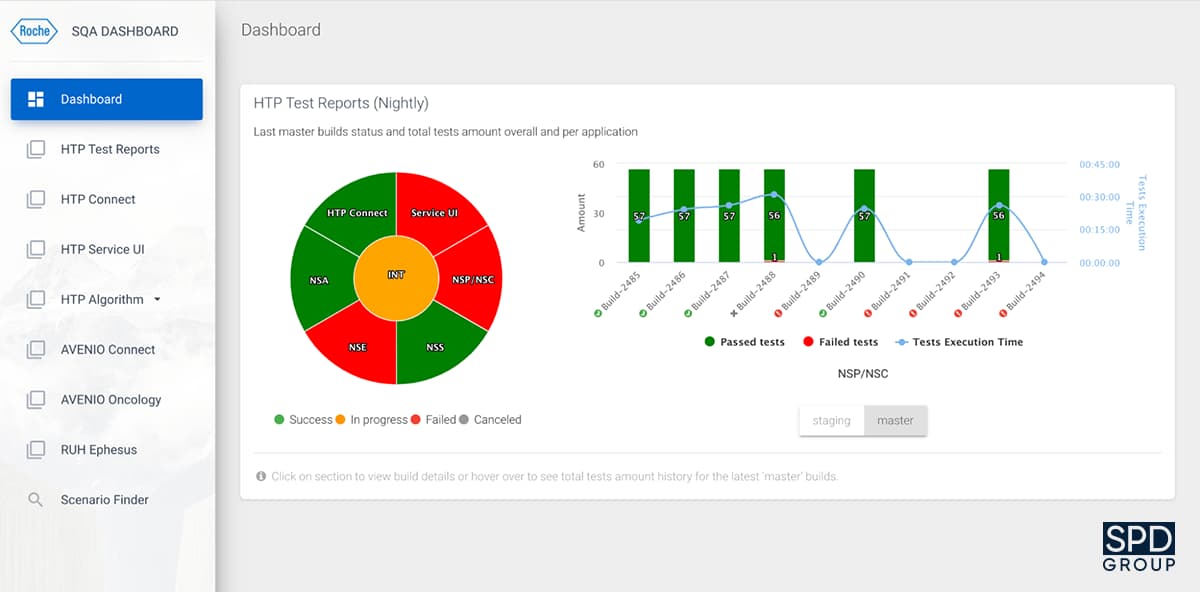
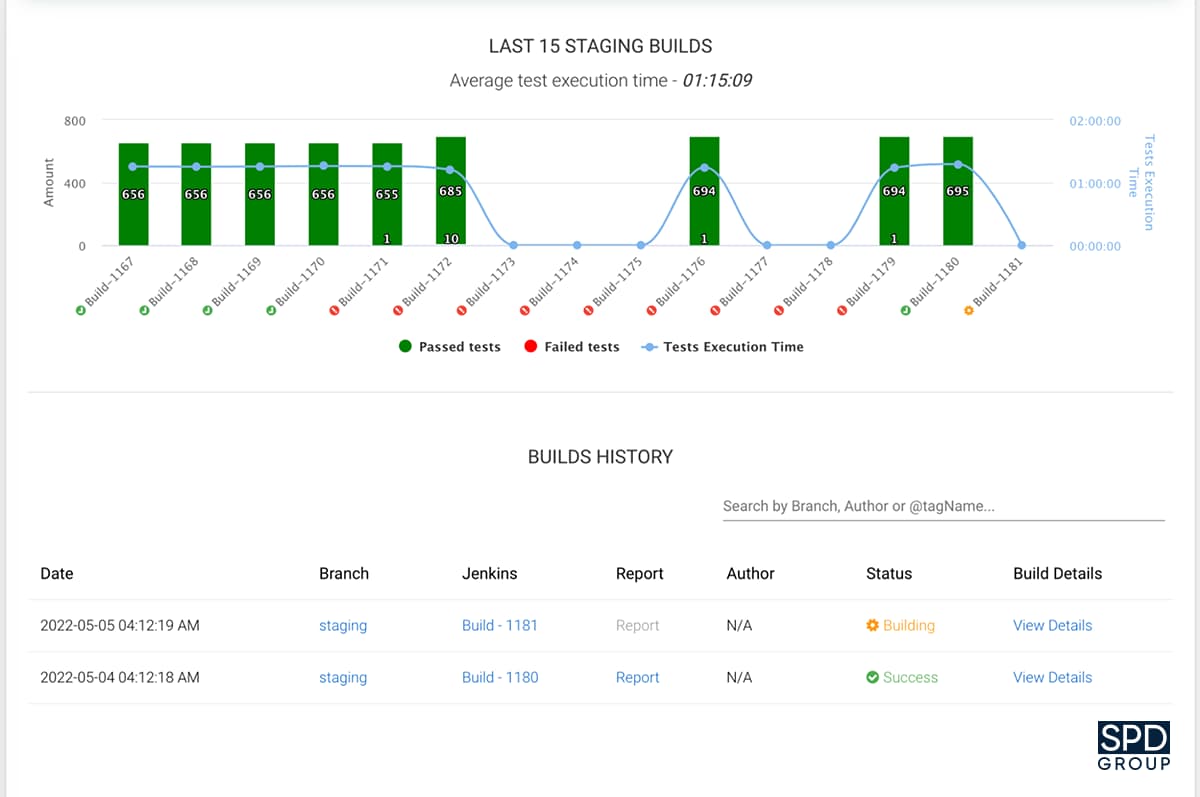
The need to use a client-defined technology stack that had also been used yet before we embarked on the project has taken us an additional effort.
Besides, the testing-related information was distributed across multiple data sources. This constituted yet another project challenge we’ve successfully overcome.
Technology Solution
- Spring Boot
- Angular
- Java 8
- GCP
- MySQL
- Git
- Auth0
Methodology, Tools:
- Kanban
- CI/CD
Result
As of February, 2023, SPD Technology’s project team has been working on Roche’s project for more than 3.5 years.
The COVID-19 pandemic-related lockdown slowed down the project’s progress, limiting our experts’ on-site access to the client’s California-based laboratories.
Currently, the project is actively under development. The members of our project team continue to provide automated and manual testing services, develop simulator software, perform business analysis, and expand the solution’s Software Quality Assurance dashboard.
We have dramatically reduced the possibility of damage to Roche’s DNA-sequencing hardware and software during the testing of edge cases, thus safeguarding and enhancing one of their major business processes.
Developing the Software Quality Assurance dashboard has improved the testing process and created significant time savings for the client’s other teams.
>Some of the automated tests our experts have created ensure 3x-4x time savings as compared with manual testing.
Ready to speed up your Software Development?
Explore the solutions we offer to see how we can assist you!
Schedule a Call